ASSESSMENT OF WILDLIFE AND LIVESTOCK DISEASE INTERACTIONS IN THE
NGORONGORO CONSERVATION AREA OF TANZANIA.
PAUL RWAMBO, JAN GROOTENHUIS, JIM
DEMARTINI AND SAMSON MKUMBO
SUMMARY AND LESSONS LEARNED
The Ngorongoro
Conservation Area (NCA) is a large area covering 8,300 square kilometers of
land supporting various numbers of wildlife species and livestock. The NCA was
designated a multiple land use area in 1959 and is divided into six land use
zones based on rainfall, vegetation, and topography. Several factors including
availability of pasture, water, and salts influence the annual livestock
grazing patterns in the NCA. The
presence of ticks and tick-borne diseases and the potential for transmission of
malignant catarrhal fever are major determinants of livestock grazing patterns, and a possible source of conflict
between pastoralism and wildlife conservation. Participatory rapid appraisals
to determine the priority diseases of livestock, the animal health constraints
to livestock productivity and the community perception to wildlife as a
potential source of diseases of livestock were conducted. In 1998, the
pastoralists identified East Coast fever (ECF), ormilo (turning sickness), malignant catarrhal fever, anaplasmosis,
contagious bovine pleuropneumonia, blackquarter, lumpy skin disease and anthrax
as the most important diseases affecting cattle, sheep and goats. Since 1984,
the incidence of tick-borne diseases including ECF and ormilo increased
drastically and the average mortality rate associated with the two tick-borne diseases
was 18% in adults and 52% in calves under 12 months of age. The risk of
transmission of diseases from wildlife to livestock was only associated with
the wildebeest. Disease incidence varies with the ecological variety, but,
because of animal movements, virtually all livestock is at risk from all
diseases present in the NCA. The information on disease interactions forms a
baseline for development of a disease model for the integrated monitoring and
assessment system (IMAS)
.
The investigations on wildlife / livestock disease
interactions in the Ngorongoro Conservation Area revealed that some wildlife
diseases and several livestock diseases constrain pastoralism and cause conflict
between livestock production and conservation of natural resources. The lessons
learned in the study include:
1. During
discussions with key stakeholders and community members during participatory
rapid appraisals, the following diseases of
livestock were identified as posing serious constraints to livestock
production in the NCA:
East Coast fever, Ormilo, malignant catarrhal fever, contagious bovine
pleuropneumonia, calf pneumonia, anaplasmosis, anthrax and blackquarter were
the priority diseases requiring urgent intervention because of the high
mortality rates they cause in livestock. An average mortality rate of 52% for
calves below the age of one year was reported. This high mortality rate in
itself could be responsible for the serious decline of cattle populations that
has been observed in the NCA for a number of years. Tick-borne diseases,
principally East Coast fever, were listed as responsible for the high calf
mortality. During the study, it became apparent that there is very little information,
if any, on cause-specific morbidity and mortality data on nearly all the
livestock and wildlife diseases in the NCA.
2. The annual
removal of livestock from the short grass plains during the wet season to the intermediate and highland areas in
avoidance of exposure to MCF virus being secreted from 2-4 months old
wildebeest calves exposes livestock to high risks of transmission of tick-borne
and infectious diseases. We were surprised to note that the community does not associate
buffalo as a source of livestock disease, particularly as a source of ECF.
3. Although the
disease risks are not evenly distributed in the NCA, the frequent migration of
livestock in search of good pasture, water, salts, markets and in avoidance of
specific diseases invariably leads to livestock being at risk of exposure to
all the wildlife and livestock diseases. The situation is worsened by the
concurrent migration of various wildlife species in search of pastures, water,
and salts. However, the risk of transmission of some diseases including MCF,
trypanosomosis, anthrax and blackquarter is confined to geographically defined
areas where risk can be mitigated by avoidance albeit at the expense of availability
of good grazing.
4. The
concentration of livestock and wildlife in the available pastures is a
potential source of conflict between pastoralism and natural resource
conservation. The available space is greatly reduced through concentration of
animals in areas with low risk of transmission of disease causing agents such
as the MCF virus during the wet season.
5. To balance
pastoralism and conservation of natural resources in the NCA there is a need to
develop a sustainable livestock management program for the control of
tick-borne and infectious diseases. A prerequisite of the development of such a
programme is the presence of a capacity to diagnose disease both in wildlife
and livestock. There is some capacity to recognize clinical disease and provide
treatment, but there is a clear lack of diagnostic ability to deal with
mortality epidemics in both livestock and wildlife.
INTRODUCTION
Arid and semi arid lands cover a large
proportion of East Africa and support pastoralism and large wildlife
populations. Interactions of infectious
and non-infectious diseases of livestock and wildlife, availability of feeds
and water resources and conservation policy are important factors that
determine human settlement, land use patterns, livestock grazing patterns and
wildlife conservation. These factors also influence community perception
towards conservation of natural resources in the pastoral ecosystems of East
Africa. Pastoralism and
wildlife conservation are complementary land use systems as both require large
ranges and have seasonal migration of animals in search of forage , salts and
water resources. Pastoralism competes with wildlife conservation. With no space
limitation there is no evidence of competition for forage (Prins, in
press).However, when space is limited competition for feed and avoidance of
disease risk become major factors in compatible livestock and wildlife
management (Grootenhuis and Olubayo, 1993). Buffalo and wildebeest are the two
wildlife species that constitute reservoirs for livestock diseases of great
economic importance (Grootenhuis, in press). Pastoral livestock production has
in the past been compatible with
wildlife conservation. More recently, competing forms of land use in pastoral
ecosystems, including livestock production, crop agriculture, tourism and
wildlife conservation, have exacerbated the potential for conflict between
pastoralism and conservation. Pastoral migration has been increasingly
restricted by game reserves, crop agriculture, land subdivision and individual
land tenure. In the areas where crop agriculture has been introduced
destruction of the crops by wildlife causes additional conflicts between
pastoralism and wildlife conservation. Co-existence of wildlife and livestock
populations provide conditions that are favorable for transmission of viral,
bacterial and parasitic disease agents among wild ruminants, cattle, sheep,
goats and camels. The resulting diseases adversely affect livestock and
community welfare and contribute to conflicts between wildlife
conservation and livestock production. Consequently, there is a need to
establish a more appropriate and sustainable balance between food security,
welfare and natural resource conservation
in the pastoral areas of East Africa. In the present study, wildlife and
livestock disease interactions and the risks for transmission of both
infectious and tick-borne diseases to livestock in the NCA were assessed using
participatory rapid appraisals and literature review. The results indicate that
diseases are crippling the viability of livestock production in the NCA.
However, with the exception of the wildebeest, wildlife is not perceived to
play a major role in the transmission of disease to livestock. Several economically
important diseases cause very high mortality in bovine calves and adults,
reducing the viability of pastoralism in the NCA. It will be necessary to
verify the role of wildlife, in particular the buffalo, as a source of
livestock disease in the NCA.
The Ngorongoro Conservation Area (NCA).
The
NCA was created in 1959 as a multiple
land use area dedicated to the promotion of both natural resource conservation
and human development. The NCA covers an area of 8,300 square kilometers, has
diverse topographical-ecological zones and varying numbers of wildlife species
and supports pastoralism. Livestock
production is the cornerstone of the Maasai economy and any reduction in
livestock productivity will ultimately affect conservation in a negative way.
The distribution of livestock and wildlife species in the NCA varies with the
seasons, vegetation, disease risk and the landscape. The diverse ecological
zones of the NCA, ranging from the Northern highland forest reserve to the short-grass plains, present varying risks
of transmission of infectious and tick-borne disease causing pathogens from
wildlife species and livestock. For sustainable management of the NCA it is
imperative that the risk of transmission of
tick-borne and infectious disease causing agents be reduced through an
improved livestock health management strategy.
Study objectives.
The information obtained in this study
will contribute to development of a model for predicting different scenarios of
possible outcomes of wildlife-livestock disease interactions in the NCA. The
NCA was selected as a study site
because of availability of data for the development of the integrated
modeling and assessment system (IMAS) and its importance as an ecologically
diverse area that supports pastoralism and natural resource conservation. Four
livestock diseases including malignant catarrhal fever, East Coast fever,
rinderpest and brucellosis were selected for model inclusion during a workshop
held in Nairobi in 1997. Following literature review and discussion with key
informants, it became apparent that obtaining information on all livestock
diseases perceived by the Maasai of the NCA as important constraints to
livestock productivity was crucial to the success of the study. All the disease
factors that contribute to reduction in productivity of livestock in the NCA
must be identified and be given important consideration if the twin goals of
human development and natural resource conservation of the NCA are to be
sustained. The study aims to review pertinent literature on livestock-wildlife
diseases and conduct field investigations using the participatory rapid
appraisals (PRAs) to address the
following issues on livestock-wildlife disease interactions in the NCA:
·
Obtain
information on livestock herd structure and population trends;
·
Determine
the common diseases of livestock in the NCA;
·
Obtain a
community perception of the priority diseases of livestock;
·
Obtain
information on household livestock mortality for a period of 18 months;
·
Assess the
constraints to effective control of livestock diseases in the NCA;
·
Determine
the ecological or geographical distribution
of risks of transmission of disease causing agents;
·
Identify
the community perception of the role of wildlife species in transmission of
diseases to cattle;
·
Obtain
information on seasonal livestock movements within the NCA;
·
Obtain
specific information on wildebeest migration, wildebeest calving and exposure
of cattle to malignant catarrhal fever (MCF);
·
Obtain
information on community mitigation strategies to reduce disease transmission,
and;
·
Obtain
disease risk data for development of disease model for incorporation in to the
IMAS model;
·
Identify
cause-specific morbidity and mortality;
·
Identify
gaps in information on disease occurrence and risks for transmission and make recommendations.
Methods.
Selection of study sites:
The study sites were selected
based on the information obtained during discussions with key informants who
included the Community Development Officer and the regional livestock officers
of the NCA. The criteria used for selection of study sites included:- 1)
livestock abundance and production, 2) human settlement, 3) land use zone, 4)
wildlife abundance and 5) accessibility by road. Based on these criteria, three
main sites including Olbalbal, Nainokanoka, and Endulen and one minor site,
Olairobi, were selected. The selected sites represent different land use,
ecological and topographical areas. The livestock officers from each area were
requested to inform and invite key participants to attend the PRA interviews.
At each site, seven to 12 livestock owners participated in the rapid
appraisals. The PRA aimed at obtaining existing veterinary knowledge on
livestock diseases, and where possible, obtain qualitative epidemiological data
on household livestock numbers (calves and adult cattle), age related mortality
and livestock sales, disease prevention and control, constraints to animal
health delivery, livestock mobility patterns,
and the association of wildlife to livestock diseases. At all the sites visited, the animal owners
were very clear with the issues they thought were important for livestock
production.
Results and Discussion.
1.
Livestock
population trends and herd composition.
As livestock production is the
major economic activity of the Maasai pastoralists it was found necessary that
data on livestock population trends be obtained. The human population in the
NCA has increased tremendously (Bureau of Statistics, 1991) without concomitant increase in the livestock population. This
trend has resulted in a reduction of the number of livestock per capita (McCabe
et al., 1989). Inadequate animal services in the NCA are blamed for the
continued reduction in livestock numbers. In our investigations, nearly all the
animal owners responded that they had fewer animals than they used to have ten
years ago. Diseases of livestock including malignant catarrhal fever that is
derived from young wildebeest calves were cited as the main cause of mortality.
Age related herd composition data was derived from the figures that were given
by the respondents. The information on the number of cattle owned by each
respondent was verified by the other participants. The results in Figure 1 show
that calves (young cattle below 12 months of age) represent 39% of the cattle population and cattle above 12
months of age represent 61% of the cattle population. This is indicative of a
good herd but the survival rate for calves is low as shown below. The
respondents were also asked to state the number of adult cattle sold for the
period 1997-98. The proportion of adult cattle sold for the period 1997-98 in
Nainokanoka area is shown in Figure 2.
2.
Dynamics of livestock diseases in the
NCA.
Co-existence of both
wildlife and livestock populations in the same ecosystem provides conditions
that are favorable for transmission of disease causing pathogens between
wildlife and livestock. Additionally, a diverse ecological setup as seen in the
NCA presents ideal situation for survival of a variety of biological vectors of
disease causing agents. Such vectors include ticks (for tick-borne protozoal
and viral diseases), mosquitoes (for Rift Valley fever virus) and tsetse (for
trypanosomosis). The distribution, the population density and the success of
survival of these vectors is determined by a variety of environmental and
Figure 1: The
herd structure of cattle population owned by respondent pastoralists
interviewed during participatory rapid appraisals conducted in the NCA. The
herd structure is shown as calves below the age of 12 months and adult cattle.
Figure 2. Mortality and sales as a proportion of the adult cattle population of
respondents Maasai in the NCA.
biotic
factors. In conditions where there is minimal movement of livestock and
wildlife the risk of transmission of vector-borne pathogens is dictated by the
vector distribution. In the NCA, the extensive movement of livestock and
wildlife, and their increased concentration at watering sites, amplify the risk
of transmission of vector-borne and infectious disease pathogens. In addition,
the concentration of livestock in bomas, also increases the risk of
transmission of vector-borne and infectious diseases and gastrointestinal
parasites. The risk of transmission of disease pathogens in the NCA thus becomes
a factor of the distribution of disease
causing agents, the movement of livestock and wildlife, the season and the
environment and livestock management. Understanding these factors and how they
interact with each other within an ecosystem would allow for a rational disease
control program, institution of the supportive policy framework and the
sustainable utilization of natural resources in the NCA. In this study, we also
assess the state of livestock production and animal health in the NCA, assess
how the risk of transmission of disease causing agents influence animal
movement, how shift from pastoralism to agro-pastoralism will influence
livestock production and natural
resource conservation. The information obtained will form a baseline for
development of a model for the simulation of disease transition states
within the target livestock populations. Such models can be used to assess the
influence of disease interventions on the profitability of livestock production
and the long term health of the ecosystem.
2.1
Common diseases of
livestock.
The data on
livestock diseases was obtained through conducting participatory rapid
appraisals aimed at getting the existing veterinary knowledge within the NCA.
The animal owners were asked to list the diseases that affect their livestock,
and where possible describe the common clinical presentation, identify the time
of the year that the disease occurs and describe past experiences with the
diseases. The diseases that the pastoralists identified as constraints to livestock
production, and which contribute to the decline in livestock population in the
NCA are listed in Table 1.
2.2
Priority ranking of
livestock diseases.
In each group, the
participants were asked to rank five most important diseases of cattle. The
criteria for ranking included mortality rates, economic losses and frequency of
occurrence of disease outbreaks. The priority ranking of important livestock
diseases is presented Table 2.
2.3
Calendar of important
livestock disease in the NCA.
The interview respondents
were asked to identify the periods when some of the important diseases affected
their livestock. An annual disease calendar, differentiating the wet and the
dry seasons, was developed and is outlined in Table 3. The information,
regardless of how precise the data is, forms a useful basis for planning any
investigations on the particular diseases mentioned. The calendar shows that
the disease problems are worst during the dry season. This implies that
strategic interventions can be applied during the wet season to avert the high
incidence of disease during the dry season.
Table 1. Diseases
affecting cattle, sheep and goats in the NCA as identified during participatory
rapid appraisals conducted in Olbalbal, Endulen and Nainokanoka.
|
DISEASE
|
OLBALBAL
|
ENDULEN
|
NAINOKANOKA
|
|
1
|
East
Coast fever
|
^
|
^
|
^
|
|
2
|
Anaplasmosis
|
^
|
^
|
^
|
|
3
|
OrmiloF
|
^
|
^
|
^
|
|
4
|
Anthrax
|
^
|
^
|
^
|
|
5
|
Pneumonia
|
^
|
0
|
0
|
|
6
|
Eye
infections
|
^
|
^
|
0
|
|
7
|
Skin
infections -mange
|
^
|
0
|
^
|
|
8
|
Acute
diarrhoea
|
^
|
^
|
0
|
|
9
|
Malignant
catarrhal fever
|
^
|
0
|
^
|
|
10
|
Rinderpest
|
^
|
0
|
^
|
|
11
|
Foot-and-mouth
disease
|
^
|
^
|
^
|
|
12
|
Contagious
bovine pleuro-pneumonia
|
^
|
^
|
^
|
|
13
|
Contagious
caprine pleuropneumonia
|
^
|
0
|
^
|
|
14
|
Bloat
|
^
|
^
|
0
|
|
15
|
Helminthosis
|
^
|
^
|
^
|
|
16
|
Coccidiosis
|
^
|
0
|
0
|
|
17
|
Blue
tongue
|
^
|
0
|
0
|
|
18
|
Haemorhagic
septicemia
|
^
|
^
|
0
|
|
19
|
Trypanosomosis
|
^
|
^
|
^
|
|
20
|
Blackquarter
|
^
|
^
|
^
|
|
21
|
Lumpy
skin disease
|
^
|
^
|
0
|
|
22
|
Babesiosis
|
^
|
^
|
0
|
|
23
|
Foot
rot
|
^
|
^
|
^
|
|
24
|
Heartwater
|
^
|
^
|
^
|
|
25
|
Brucellosis
|
^
|
^
|
^
|
|
25
|
Nairobi
sheep disease
|
0
|
0
|
^
|
|
|
|
|
|
|
Table 2. Priority
ranking of livestock diseases by Maasai
respondents during participatory rapid appraisals conducted in three sites in
the NCA
|
OLBALBAL
|
ENDULEN
|
NAINOKANOKA
|
|
1
|
East
Coast fever
|
East
Coast fever
|
East
coast fever
|
|
2
|
Malignant
catarrhal fever
|
Ormilo
|
Ormilo
|
|
3
|
Calf
pneumonia
|
CBPP
|
Anaplasmosis
|
|
4
|
Anaplasmosis
|
Blackquarter
|
Blackquarter
|
|
5
|
Anthrax
|
Lumpy
skin disease
|
Malignant
catarrhal fever
|
Table 3: Seasonal calendar of livestock diseases
in the NCA. The disease calendar was derived from information obtained during
PRAs in Nainokanoka, Ol BalBal and Endulen in 1998.
|
JAN
|
FEB
|
MAR
|
APR
|
MAY
|
JUN
|
JUL
|
AUG
|
SEP
|
OCT
|
NOV
|
DEC
|
|
|
|
WET SEASON
|
DRY SEASON
|
WET SEAS.
|
|
|
ECF
|
ECF
|
|
ORMILO
|
ORMILO
|
|
|
|
|
|
|
ANAPLASMOSIS
|
|
|
|
|
|
|
|
BLACK-QUARTER
|
|
|
BRUCELLOSIS
|
|
|
|
BRUCELLOSIS
|
|
CBPP
|
CBPP
|
CBPP
|
|
|
|
FOOT-AND-MOUTH DISEASE
|
|
|
|
|
|
|
|
|
MCF
|
|
|
|
|
|
|
|
ANTHRAX
|
ANTHRAX
|
|
|
|
|
LSD
|
|
WORM INFESTATION
|
|
|
|
|
|
|
|
|
|
|
|
|
|
DISEASE KEY
|
|
Disease most serious
|
|
|
Disease less serious
|
2.4. Household
distribution of cattle and mortality rates.
The per household distribution of livestock
varied greatly as shown in Figures 3 and 4 on mortality rates in calves and
adult cattle. The participants were asked to state the number of calves born
and the number of calves that died as a result of infection with diseases. The
accuracy of the information was verified by triangulation and by the
participants knowing the herd sizes of each other. The groups classified cattle
below 12 months as calves. On the horizontal axis the individual households are
listed randomly from 1-12, the white bars indicate the total number of calves
present and the black bars indicate the number of calves died over the 18
months period (‘97-’98). The data show that there is great variation in the
number of cattle per house-hold and the
annual mortality rates in calves. This variability may reflect differences in
the level of livestock management. As shown in Figure 3, the mortality rates in
calves was very high ranging from 16-75%.
The average calf mortality was 52%. This is an alarming mortality rate
and supports the observations on the serious decline of cattle populations in
the NCA for a number of years. Tick-borne diseases, and principally East Coast
fever, were responsible for the high calf mortality in Nainokanoka. Calf
mortality in the other sites was also very high and at Endulen mortality rates
of 50-60% were reported.
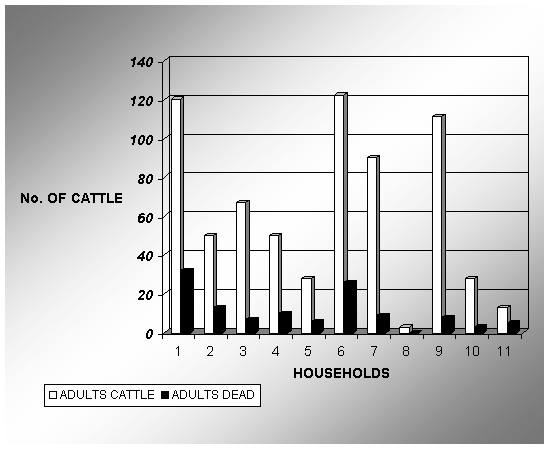
The number of adult cattle per household
varied markedly (Figure 4). The mortality rate was not as high as that observed
in calves and varied from household to household. The average mortality rate
for cattle owned by the participants for a period of 18 months was 18%. During
the interviews, it was quite evident that the pastoralists have enormous
information on livestock diseases including wildlife diseases transmitted to
cattle. However, it is important to point out that there was no information on
cause-specific mortality in any of the livestock. There was no quantitative
data on disease incidence and mortality based on clinical and serological
diagnoses and necropsy of dead livestock. Although the qualitative
epidemiological information obtained from the animal owners clearly identifies
the animal health issues there is need to conduct research on the priority diseases to identify the risk
distribution, mechanisms of transmission and possibility of mitigating against
disease transmission.
Figure 3. Household
calf numbers and mortality rates for a period of 18 months (Jan ’97 to July
’98). The data was provided by respondents during a participatory rapid
appraisal conducted at Nainokanoka in the NCA. On the horizontal axis the
individual households are randomly listed form 1-12. White bars indicate the
total number of calves present during the 18 months period, while black
bars indicate the number of calves
died. The mortality ranged from 16-75% averaging 52%.
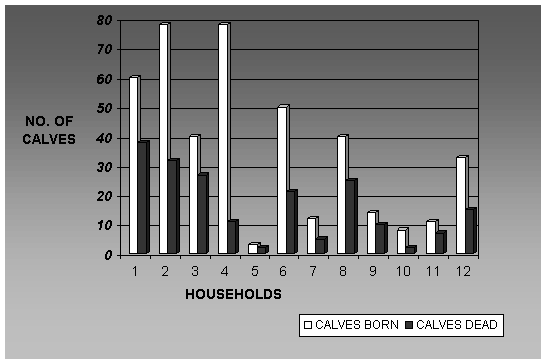
Figure 4: Household
distribution of adult cattle and mortality rates for a period of 18 months.
The data obtained during a
participatory rapid appraisal conducted in Nainokanoka. On the horizontal axis
the individual households are randomly listed form 1-11. White bars indicate
the total number of adult cattle present during the 18 months period, while
black bars indicate the number of
cattle died. The mortality ranged from 6-38% averaging 18%
3 Wildlife / Livestock disease interface.
3.1 Wildlife - livestock movements and diseases.
Pastoralists in the NCA move their
livestock for a variety of reasons. As mentioned earlier, increased movement of
livestock and wildlife have serious consequences to animal health. Migratory
wildlife species, especially wildebeest and zebra, migrate in to the short
grass plains of central and eastern Olduvai during the wet season. From
December to February, wildebeest move from Serengeti, south-eastwards into
Ngorongoro through Oduvai and Olbalbal areas. The migration coincides with the calving of most of the
NCA wildlife, including the wildebeest. The wildebeest move back into Serengeti
through the same route in April, May and June. The migration of wildebeest into
and out of the NCA is a major factor that dictates the movement patterns of
livestock as the risk of transmission of malignant catarrhal fever (MCF) virus
from young wildebeest calves precludes the coexistence of cattle with wildlife
in the short grass plains during the wet season. Early in the wet season, the Maasai and their livestock
move from the plains to the woodlands areas in order to avoid the wildebeest on
the plains. This way, the pastoralists
attempt to avoid the MCF risk associated with wildebeest during the calving
season.
The other factors that influence
livestock migration in the NCA included:
·
Inadequate
resources for feeding livestock; grazing pastures influenced by seasons;
·
Inadequate
water for livestock;
·
Natural
mineral supplements for livestock;
·
Avoidance
of risks of disease transmission;
·
Marketing
of livestock;
·
Cattle
rustling.
During the interviews, it was learnt that
the ongoing outbreak of CBPP might have originated from cattle brought from
Shinyanga. Such movement of livestock, especially when disease control is weak
or non-existent, will in most cases lead to emergence of livestock diseases. In
the dry seasons, livestock is moved from the slopes to the highlands where good
pastures are available. This exposes the lowland livestock to the risks of
transmission of tick-borne diseases. To the communities, the benefit derived
from livestock having access to good pastures exceeds the risks or costs of
transmission of tick-borne diseases.
3.2 Role of wildlife in
transmission of diseases to livestock.
When asked about the role of
wildlife in the transmission of disease causing agents to livestock the
respondents could only associate the wildlife to malignant catarrhal fever in
cattle. It was reported that rinderpest last occurred in 1983 and caused a high
mortality in buffalo. With the success achieved by the Pan-Africa Rinderpest
Campaign (PARC) rinderpest is currently not a problem in the NCA. Although
buffalo-derived strains of Theileria
parva have been known to cause serious disease in cattle in other parts of
East Africa the respondents had no association of buffalo to any disease risk
for cattle. The close contact between wildlife and livestock implies that
several major diseases and vectors of disease causing agents can be shared and
pose a constant threat to livestock. The following diseases can be associated with
wildlife species:
Table 4: The most important diseases and vectors are
listed with indications if the disease is transmitted from domestic animals to
wildlife (C>W) or the other way round (W>C), or if the disease is
maintained in wildlife and domestic animals (W+C) or in livestock alone (C).
Diseases: C*>W* W>C W+C C
·
Malignant catarrhal fever - + - -
·
Rinderpest + - - +
·
East Coast fever - + + +
·
Ormilo - ? ? +
·
Anaplasmosis ? + + +
·
Babesiosis - - - +
·
Heartwater - (+) + +
·
Trypanosomosis + + + +
·
Brucellosis + - + +
·
Foot-and-mouth disease + + + +
·
Intestinal parasites - - + +
·
Rabies + - - +
·
Anthrax - - - -
Vectors:
·
Ticks
·
Tsetse
·
Mosquitoes
*C= cattle or livestock, including
domestic dogs in the case of rabies. W= wildlife. > indicates the direction
of transmission. + there is transmission as indicated, - no transmission. ?
transmission may occur, but no good data available, (+) transmission does
occur, but may not play a major role in the epidemiology.
The
occurrence and distribution of these diseases and vectors depends on several
factors including: host population densities; degree of interaction between
wildlife and livestock species; herd immunity and availability of susceptible
members of the host population; availability and survival of the disease
causing agents; availability, population density and survival of infectious
vectors. In Table 4 it can be seen that only MCF is maintained only in
wildlife, being wildebeest, and a large
number of diseases are maintained both in wildlife and cattle.
4. Epidemiological information of major livestock diseases in the NCA.
This activity
was carried out by: 1) conducting interviews and discussion with personnel involved in animal health,
diagnosis and policy; 2) livestock owners; and by 3) reviewing documents and published literature. The target diseases selected at the onset of the
project were malignant catarrhal fever, rinderpest and east coast fever. Field
visits and PRAs conducted in the NCA in 1998 revealed that the priority
diseases, in terms of high mortality and economic losses, did not include
rinderpest and brucellosis. Though rinderpest causes high mortality in
non-immune cattle, recent vaccinations
co-ordinated by the Pan-African Rinderpest Campaign (PARC) have helped
to eliminate rinderpest from the NCA. Consequently, animal owners do not
currently view rinderpest as a constraint to pastoralism. Although brucellosis was not ranked highly
by the pastoralists it is important to note that this disease has serious human
health consequences. The importance of a zoonotic disease, such as brucellosis,
should not entirely be viewed in terms of its mortality rate in livestock. In
fact, during the field visits, a few cases of brucellosis in humans were
reported. Understanding the magnitude and the transmission dynamics of
brucellosis would help in designing strategies of reducing the rate of
infection in pastoralists. Rabies is another disease that claims an unspecified
number of people every year. There is no systematic control of the disease
which is still only maintained by a biotype of the virus that is maintained in
dogs. There is the opportunity of controlling the disease and preventing the
adaptation to wildlife, which will result in wild biotypes of the virus not
amenable to control by vaccination of domestic dogs. This disease threatens
also wild carnivore species and has contributed to the serious decline of the
wild dog (Lycaon pictus). The
priority diseases of livestock
identified during our field visits to
three land use zones where cattle rearing is a major activity are as
summarized in Table 1. The occurrence of some diseases, such as malignant
catarrhal fever, is confined to areas where cattle co-graze with wildebeest
calves. Our study, in general, revealed that accurate information on disease
transmission and cause specific mortality, is not available.
5. Risk assessment of disease transmission
to livestock in the NCA.
An assessment of the risks associated
with disease transmission amongst livestock and between livestock and wildlife
was conducted in 1998. The disease risks were analyzed according to the mode of
transmission, geographical distribution, and involvement of wildlife species as
reservoirs of disease causing pathogens. The risk of livestock and wildlife
contracting disease depends on the distribution of pathogens, vectors, hosts
and the dynamics of their interactions as illustrated in figures 5 and 6.
Figure 5 shows how vectors, livestock, wildlife, people may interact with a
variety of diseases in the same ecosystem. More specific information on
wildlife / livestock disease interactions is given in Table 4. The distribution
of Rhipicephalus appendiculatus, ECF in cattle, buffalo and the risk of
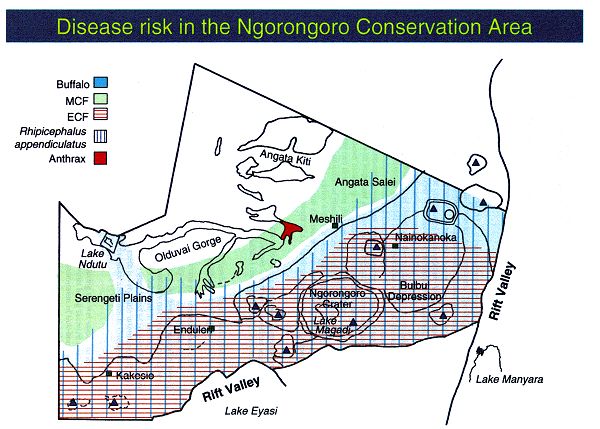
exposure to MCF are depicted in a map of the NCA to illustrate risk
distribution for these two diseases (Figure 6). The risk for MCF depends only
on the presence of infectious virus. Wildebeest calves up to the age of three
months are the source of infectious virus. The risk is seasonal and limited to
the distribution of this age class
of wildebeest calves. In the NCA the highest risk of contracting
MCF is between the months of January and March. The area where the cattle show
signs of the disease (incidence) may not have any relationship with the area
where they were exposed, because of cattle movement during the incubation
period. In this case disease risk and disease incidence have no clear
geographic relationship. In contrast, ECF cases are usually confined to the
areas where the disease is contracted from infected R. appendiculatus ticks.
Table 5: Geographic
distribution, the risk of transmission and the intervention strategy for
control of common livestock diseases in the
NCA.
|
|
Diseases
|
Geographic distribution
|
INTERVENTION
|
|
1
|
Tick-borne diseases
·
East Coast fever (ECF)
·
Ormilo (turning sickness)
·
Anaplasmosis,
·
Babesiosis ,
·
Heartwater
·
Nairobi sheep disease.
|
·
Highlands,
·
Crater,
·
Slopes,
·
Woodlands
High humidity and vegetation cover. May be
widespread risk because animal movement.
|
·
Early treatment with butalex;
·
Infection and treatment with local
parasite strains;
·
Improved tick control with acaricides.
|
|
2
|
Transboundary
diseases
·
FMD;
·
CBPP;
·
CCPP;
·
Lumpy skin disease (LSD).
|
Not geographically defined. Risk increased by
uncontrolled animal movement.
|
·
Vaccination;
·
Vaccination and surveillance;
·
Vaccination and/or antibiotics;
·
Vaccination.
|
|
3
|
Point source
diseases
·
Anthrax;
·
Blackquarter.
|
Risk confined to
limited areas for example in the Olbalbal swamp and depression.
|
·
Vaccination
·
Vaccination.
|
|
4
|
Wildlife diseases:
·
MCF;
·
Trypanosomosis;
·
FMD;
·
Brucellosis
·
Tick-borne diseases;
·
Intestinal parasites.
|
·
MCF risk confined to short grass plains
from Jan-April.
·
Tryps confined to low woodlands and
riverine areas.
|
·
Keep cattle away from WBs.
·
Chemoprophylaxis;
·
Vaccination of cattle;
·
Vaccination;
·
Reduce ticks with acaricides;
·
Strategic worming of cattle.
|
|
5
|
Gastrointestinal
parasites.
|
Bomas
|
·
Strategic use of anthelminitics.
|
|
6
|
Bacterial pneumonia
|
Bomas and highlands
|
·
Antibiotic treatments
|
Note: Because
of the frequent movement of wildlife
and livestock, virtually all livestock is at risk of all the diseases in the
NCA.
Tick-borne diseases
East Coast fever was
recorded as the most serious and widely distributed disease of cattle in the
NCA. The most severely affected areas are Nainokanoka, Endulen, Esere, Kakesio
and part of Olairobi. Many deaths in calves,
yearlings and adults are observed. During the dry season, Ormilo (a
disease of cattle manifested by CNS involvement) is the most serious tick-borne
disease. The relatively low wildlife population in the high livestock
utilization areas suggests that involvement of wildlife in the transmission of
ECF to livestock is minimal. The risk factors associated with transmission of
tick-borne parasites include:
5.1 A high population of ticks in the medium level and
highland areas of the NCA enhanced by the lower temperatures, high humidity and
good vegetation cover. These factors favor tick species diversity, high
population density and tick distribution and survival. Frequent movement of livestock
in search of pasture, water, salts and markets enhance tick dispersion and the
risk of transmission of tick-borne and infectious diseases. Livestock that are
moved into the plains in search of good pasture, water and salts have to be
moved back to the medium to high lands to avoid transmission of MCF from young
wildebeest calves. To the pastoralist, fear of the decimating effects of MCF
far outweighs the risk of transmission of tick-borne diseases in the highlands.
The risk of transmission of tick-borne
diseases is increased by the absence of
adequate and effective tick control program within the NCA.
5.2 A high diversity of Theileria parva parasite
populations. Although there is no
molecular evidence currently is support of genetic and antigenic diversity of
parasite in the NCA, research at ILRI has that in other areas in East Africa
there is a wide diversity of
immunologically distinct Theileria
parva parasites that circulate within
an ecosystem. It can be assumed that a similar parasite diversity obtains in
the NCA leading to high mortality in cattle and delaying emergence of enzootic
stability in the predominantly zebu cattle population in the NCA. In other
endemic areas of East Africa, endemic stability was established, such as
reported for the Trans Mara region of Kenya. It is possible that the high
mobility and high turnover by sales and mortality did not allow such endemic
stability to develop in the NCA. T
5.3 The interaction of cattle and buffaloes in the NCA poses a serious
danger of transmission of virulent buffalo-derived strains of Theileria parasites. During interviews
with animal owners it was observed that the community did not associate buffalo
with possible transmission of tick-borne diseases, particularly ECF. The
community could only, on a historical basis, associate the buffalo with
rinderpest. The risks of transmission of ECF parasites from buffaloes to cattle
could be confounded by the high incidence of ECF in areas where buffaloes have
not grazed for long periods of time. There is need to investigate the role of
buffaloes in the high incidence of Theileria infections and the absence
of endemic stability to ECF in the NCA.
To balance pastoralism and conservation of natural
resources in the NCA there is a need to develop a sustainable livestock
management program for control of tick-borne diseases. The involvement of the
communities in program formulation, financing, and management would immensely
contribute towards a sustainable control of theileriosis (ECF and Ormilo). The
control of theileriosis in the NCA would
improve on house-hold welfare through reduction on calf mortality and an
increase in adult cattle population
available for marketing.
Malignant catarrhal
fever (MCF).
Caused by
alcelaphine herpesvirus-1 (AHV-1), malignant catarrhal fever has remained a
major factor that has for a long time influenced the lifestyles and grazing
patterns of pastoralists in the NCA. Our investigations revealed high levels
of awareness of the risks associated with wildebeest (WB) migration and
calving. Over the years, the pastoralists have evolved grazing strategies that
generally avoid the transmission of the MCF virus to cattle. Occasionally, some
outbreaks of MCF occur in the NCA. The outbreaks could be associated with
delayed evacuation from the plains during the wildebeest calving season.
Although the risk of disease transmission from wildebeest is widely known within the community there
is almost a mythical understanding of
the critical source of the virus that
is infective to cattle. The majority of pastoralists associated MCF in cattle
with ingestion of hair shed from young
wildebeest calves. The pastoralists indicated that they found hard hair balls
in the omasum of cattle that died from MCF. Because of this post-mortem
finding, the Maasai view the ingestion of hair from wildebeest calves as
critical in virus transmission to cattle. Other animal owners thought that the
virus is transmitted from the placenta of wildebeest cows through surface
water. Cattle become infected with the MCF virus upon drinking such water.
Obviously, there are many stories on how individual herdsmen believe the virus
is transmitted from wildebeest to cattle. There is consensus that wildebeest
calving is associated with outbreaks of
MCF in cattle. The Maasai, many veterinarians and livestock owners are not
aware that the virus is transmitted from wildebeest calves during the first
three months of their lives. There is need to disseminate known information on
the transmission of the MCF virus. Such information will help to improve on the
indigenous coping strategies of avoiding the decimating effects of MCF in
cattle. Accurate information on the duration of the risk of transmission of MCF
virus to cattle will help to reduce the costs of avoidance as currently
practiced.
Malignant
catarrhal fever is an acute disease of cattle that is characterized by high
fever, severe inflammation and degeneration of the mucosae of the upper
respiratory and alimentary tracts, blindness, enlarged lymphnodes especially
those of the forehead and neck and central nervous involvement of affected
cattle. The clinical findings in the head-and-eye form, the most common form of
MCF in cattle, are readily recognizable. The affected cattle have high fever,
are restless and anorexic, and have profuse nasal discharge that may hang from
the nostrils and ocular discharges that leave a trail of matted hair on the
cheeks, a bilateral corneal opacity
that appears on the 3rd day of the disease and eventually leads to blindness, severe blockage of the
upper respiratory tract leads to noisy breathing and dyspnea. These signs
including enlargement of the lymphnodes and central nervous involvement (fine
muscular tremors, incoordination, twitching of the ears and even torticollis)
allow for differentiation of MCF from mucosal disease and rinderpest.
Important considerations for transmission of MCF virus to
cattle:
·
Wildebeest-derived acelaphine herpesvirus type 1
(AHV-1) is the predominant cause for MCF in cattle in East Africa. Though
sheep-associated herpesvirus has been documented (Mirangi et al, ),
epidemiological data on MCF epizootics preclude sheep playing any significant
role in MCF epidemics in cattle populations in East Africa;
·
Adult wildebeests are persistently infected with AHV-1
and a proportion of calves become infected with the herpesvirus
transplacentally. MCF virus has been isolated from the spleen of wildebeest
fetuses and from one week old calves indicating transplacental infection. The
infection rate in wildebeest calves, as determined by virus isolation in
cultured cells, is highest (31%) during the first 3 months of life and declines with age to almost 2% during the 4th
trimester (Plowright, 1965b).
·
The seasonal occurrence of MCF in cattle is
attributable to the greater frequency and quantity of virus excreted in the
nasal secretions of wildebeest calves undergoing initial infection in the first
3-5 months of life (Rweyemamu et al., 1974). MCF virus occurs in nasal and
ocular secretions of young wildebeest calves in a stable, cell-free state. Such
cell-free virus is not found in the secretions of MCF infected cattle and adult
wildebeest (Mushi and Rurangirwa, 1981).
·
Cattle become infected through contact with cell-free
virus excreted in the nasal exudates of viremic wildebeest calves below five
months of age (Plowright, 1965a); Mushi et al give evidence that effective
duration of virus secretion is probably below four months of age. Calves
infected before birth are probably the most important source of virus for the
environment. The immune response in the calves reduces the amount of virus shed
in the environment from the second month of their lives and may be responsible
for switching virus shedding calves to non shedding carrier animals. This gives
only a period of one to three months of risk for cattle. These facts, although
not unlikely, need to be verified by further research.
·
The MCF virus is rapidly inactivated in the environment
through a combination of ultra-violet light and high temperature. The half-life
of the virus at 37OC is about 9 hours. The virus inactivation is
presumably faster in the high ambient temperatures obtaining in the arid and
semi-arid ecosystems of East Africa. In direct sunlight the virus is killed
within an hour, while protected by a UV filter, the virus survives much longer
(Rossiter, 19 ). The rapid inactivation of the virus in the pastures indicate
that effective virus exposure to cattle must occur within a short period; hours
not days. The amount of virus present in the pastures depends on the number and
density of wildebeest calves secreting cell-free virus and the amount of virus
in the secretions. The duration that the virus persists in the pastures depends
on the quantity and the environmental
conditions (temperature, ultraviolet radiation, humidity and vegetation cover).
·
The placenta of wildebeest cows does not play a role in
the transmission of MCF virus; no virus has been isolated from the wildebeest
placenta (Rossiter, 19 ). Similarly, the hair shed from wildebeest calves as
they change from brown to gray hair color is not involved in virus
transmission. The shedding of the hair occurs at 3 months of age when the
calves are also shedding virus in nasal secretions. This overlap or coincidence
has led the Maasai to believe that consumption of hair from wildebeest calves
by cattle leads to MCF.
·
Removal of cattle from the areas where wildebeest
calves below the age of four months are grazing eliminates opportunities for
transmission of wildebeest -derived MCF virus.
The impact of MCF on
human welfare, land use, conservation of natural resources and transmission of
diseases of livestock is enormous. The high mortality rate of MCF in cattle
reduces cattle populations and human wealth. In certain cases, wildebeest
migration has been thwarted by the use of 10-20km long fences built from thorn
trees. Such a strategy has serious consequences for the ecosystem integrity, is
not sustainable and causes habitat degradation. The movement of cattle from the
plains, at a time when pasture and water are most abundant, forces them to
graze on poor quality pastures resulting in loss of condition, starvation and
the exposure to a range of diseases not prevalent in the lowland area. The
movement of livestock from the well
pastured lowlands increases the risks of transmission of livestock diseases,
especially tick-borne diseases. The concentration of livestock and wildlife in
the available pastures is a potential source of conflict between pastoralism
and natural resource conservation of the NCA. Given that the pastoralists are
confined to the pasture available in the NCA, the cost of avoiding MCF is an
important source of conflict between pastoralism and conservation.
Constraints to effective
delivery of animal health and disease monitoring.
During the participatory rapid
appraisals conducted at Ol BalBal, Endulen, Naionokanoka and Olairobi, a number
of constraints that affect disease diagnosis, effective treatments and disease
control were raised. The constraints are summarized in Table 5. For sustainable
pastoralism in the NCA these constraints have to be urgently addressed. Decentralized animal health
delivery should be considered as an effective way of amplification of health
delivery at the community level. Other mechanisms of privatized animal health delivery
should be explored as sustainable options.
TABLE 6. Constraints to effective control of
livestock diseases in the NCA.
|
FACTORS CONTRIBUTING TO
WEAK CONTROL OF LIVESTOCK DISEASES IN NCA
|
|
|
FACTOR
|
CAUSE
|
|
1
|
Inadequate disease
diagnosis
|
1.Lack of veterinary
services.
2.Inadequate
laboratory services.
|
|
2
|
Inadequate disease
surveillance
|
1.Lack of veterinary
services.
2.Inadequate
laboratory services.
|
|
3
|
Inadequate tick
control
|
1.Lack
of knowledge on appropriate tick control methods.
2.High cost of acaricides.
3.Inadequate dips / services.
4.Inadequate water.
5.High
tick populations, wide distribution of ticks.
|
|
4
|
Transmission of
diseases from wildlife to cattle:
Malignant catarrhal
fever, trypanosomosis, possibly some tick-borne diseases.
|
1.Co-grazing of wildlife and livestock.
|
|
5
|
Inadequate
prevention and treatment of diseases.
|
1.High cost of drugs
and vaccines.
2.Pastoralist unwilling to buy drugs.
3.High disease
challenges.
|
|
6
|
Migration of
livestock
|
1.In search of
pasture, water, salts.
2.Livestock marketing.
3.Avoiding MCF.
|
References
Anon. (1988) Results on the preliminary
survey on major livestock diseases in the NCA. Report by SUA-NCAA, Norwegian Agency
for International Development (NORAD) and the Ministry of Natural Resources and
Tourism. Pp 19
De Vos, A.J. , Bessenger, R. and Banting,
L.F. (1981) Theileria ? taurotragi: a probable agent of bovine cerebral
theileriosis. Onderstep. J. Vet. Res. 48, 177-178.
Field, C.R.,Moll, G., Ole Sonkoi, C
(1988) Livestock Development. Technical Report 1, Ngorongoro Conservation and
Development Project. Pp 37
Grootenhuis, J. G. (in press) Wildlife,
livestock and animal disease. In: Wildlife Conservation by Sustainable Use. Eds.
H.H.T. Prins, J.G. Grootenhuis and T.T. Dolan. Wolters Kluwer Academic
Publications, Boston, USA.
Grootenhuis, J. G. , Morrison, W. I. , et
al (1980) Fatal theileriosis in eland (Taurotragi oryx). Pathology of natural
and experimental cases. Res. Vet. Sci. 29, 219-229.
Grootenhuis, J. G. and Olubayo, R. O.
(1993) Disease research in the wildlife livestock interface. Vet. Quart. 15,
55-9
Machange, J. (1988) Livestock Wildlife
Interactions. Ngorongoro Conservation and Development Project. Technical Report
4, pp 43
McCabe, J.T., Schofield, E.C., Pederson,
G.N., Lekule, A., and Tumaini, A. (1989). Food security and nutrition among the
Maasai of the Ngorongoro Conservation Area. Ngorongoro Conservation and
Development Project Technical Report No. 10. Nairobi:IUCN Regional Office for
Eastern Africa.
Mettam, R. W. M. and Carmichael, J.
(1936) Turning sickness, a protozoan
encephalitis of cattle in Uganda: its relationship with East Coast fever.
Parasitology, 28, 254-283
Moll, G. ,Agan, L. and Lohding, A (1985)
Bovine cerebral theileriosis. In: Immunity against theileriosis. Ed. A. D.
Irvin, ILRAD.
Mushi, E. Z., and Rurangirwa, F.R.
(1981). Epidemiology of bovine malignant catarrhal fever, a review. Veterinary
Research Communications, 5 – 127-142.
Plowright W. (1965a). Malignant catarrhal
fever in East Africa. II. Observations on wildebeest calves at the laboratory
and contact transmission of the infection to cattle. Res. Vet. Sci. Vol. 69-83.
Plowright W. (1965b). Malignant catarrhal
fever in East Africa. I. Behaviour of the virus in free-living populations of
blue wildebeest (Gorgon taurinus taurinus,
Burchell). Res. Vet. Sci. Vol. 6, 56-68.
Prins, H. H. T. (in press) Competition
between wildlife and livestock in Africa. In: Wildlife Conservation by
Sustainable Use. Eds. H.H.T. Prins, J.G. Grootenhuis and T.T. Dolan. Wolters
Kluwer Academic Publications, Boston, USA.
Rweyemamu, M.M., Karstad. L., Mushi,
E.Z., Otema, J.C., Jessett, D.M., Rowe, L., Drevemo, S., and Grootenhuis, J.G.
(1974). Malignant catarrhal fever virus in nasal secretions of wildebeest: A
probable mechanism for virus transmission. J. Wildl. Dis. 10:478-487.