|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
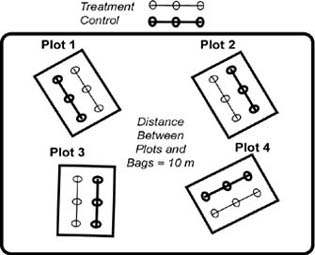
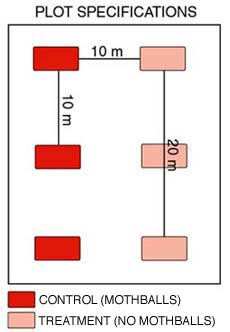
A plot is defined as two paired 20 m transects with 3 treatment and 3 control bags along each transect. Bags should be placed 10 m apart on these two parallel transects (Figure 2). Transect orientation within a plot will be randomly assigned (0-360 degrees) on-site by a random number generator. The treatment and control transects will be at least 10 m apart and parallel. This distance will address three concerns, 1) the control bags (mothballs) will be far enough from the treatment bags to avoid contamination, 2) reduce impacts of disturbance by small mammals, and 3) reduce the effects of spatial autocorrelation. Back to the top.
Mothball Application: A standardized formulation of mothballs will be added to bags at sampling time 0 in crystalline form at all sites. Mothball formulas vary internationally, so mothballs will be shipped from BioTrack in Australia. Two mothballs should be added around each control bag at each sampling time (Table 1). Back to the top. Table 1. Addition of mothballs to treatment litterbags
Barcodes to identify litterbags and specimens taken from each site will be provided by BioTrack (Mark Dangerfield, Key Centre for Biodiversity and Bioresources, Department of Biological Sciences, Macquarie University, NSW 2109 Australia). Metal id tags for barcodes will be placed underneath the bags to reduce the chance of attracting birds and mammals. Back to the top. There will be three sampling times (Table 2), and sampling interval starting points will vary with local climate. Tropic sampling (1, 2, and 3 months) will begin at the end of the wet season when plant production is at its maximum. Temperate, boreal, and arctic sampling (2, 4, and 12 months) will begin midsummer. Ideally, weather conditions are similar for sampling times within a site, but this is not probable or realistic due to seasonal variation. Site managers will record climatic data as often as possible over the sampling period. Site managers will also be responsible for site security (mammalian damage, human disturbance, etc.). Back to the top. Table 2. Sampling intervals for tropical, temperate, arctic, and boreal climates.
Litterbag Collection and Extraction Protocols: Control bags will be photographed prior to collection with id tag in clear view. The surface of bags will be carefully cleaned of adhering soil, living plant parts (roots or moss), rock fragments, etc., immediately before collection. Retrieved bags will be placed gingerly in a plastic ziplock bag, sealed, and carefully (without much disturbance) returned to the laboratory. It is crucial to ensure that decomposing materials do not fragment and fall out of the bags during retrieval or before processing. Any fragmentation or loss of material must be documented on the data sheet for each sample (Harmon et al. 1999). All bags will immediately be weighed to a thousandth of a gram and then placed gently on a Tullgren funnel. Equipment (bulb wattage, screen size), processing time (minimum of 5 days), and extractant information for dry heat extraction have been standardized by M. Dangerfield and disseminated with barcode and protocol information by mail and on the website. The recommended heating regime will achieve a residual moisture content of 10% within 48 hours. Dry faunal extractions will be followed by on-site specimen preservation with a barcode label in 10 ml vials of 95% alcohol. Vials will be provided by BioTrack. Specimens are to be shipped to BioTrack in box labeled "dead insects for scientific research - no commercial value-preserved in 95% alcohol" where they will then be identified to morphospecies for all taxa. Earthworms, termites, and water-based animals (nematodes and tardigrades) will not be sampled with this design. Following each faunal extraction, treated litter will then be oven dried, weighed, and shipped to the NREL for appropriate chemical analyses as outlined in Harmon et al. (1999) and archived on-site due to quarantine restrictions. Back to the top. This worldwide webpage will be used as a centalized database for data entry and analysis, discussion topics/papers, site-specific sampling times as well as bag handling, data collection/handling/ management, and shipping protocols. Site managers will be able to download standardized data sheets from this website in Excel format. These raw data sheets will be posted on the web, along with final morphospecies identification from BioTrack. Final data collation will be in BIOTA database format at GLIDE headquarters. This webpage will also facilitate other communications between site cooperators, Committee members, and the Project Coordinator at CSU. Back to the top. Harmon, M.E., K.J. Nadelhoffer,
and J.M. Blair. 1999. Measuring decomposition, nutrient
turnover, and stores in plant litter. Pages 202-240 in G.P.
Robertson, C.S. Beldsoe, D.C. Coleman, and P. Sollins,
editors. Standard soil methods for long-term ecological
research. Oxford University Press. Back
to the top. |
GLIDE
Please contact the GLIDE headquarters (email: glide@nrel.colostate.edu) if you have any comments or questions. GLIDE was a project of the International Biodiversity Observation Year 2001-2002 This material is based upon work supported in part by the National Science Foundation under Grant No. 98 06437 Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation. |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Home
• Research
• Publications
• Personnel
• Images
• Data
• Links |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||